| Tag | Content | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
SG ID |
SG00000107 |
|||||||||||||||
UniProt Accession |
||||||||||||||||
Theoretical PI |
5.64
|
|||||||||||||||
Molecular Weight |
80886 Da
|
|||||||||||||||
Genbank Nucleotide ID |
||||||||||||||||
Genbank Protein ID |
||||||||||||||||
Gene Name |
mlh1 |
|||||||||||||||
Gene Synonyms/Alias |
||||||||||||||||
Protein Name |
||||||||||||||||
Protein Synonyms/Alias |
SubName: Uncharacterized protein |
|||||||||||||||
Organism |
Danio rerio (Zebrafish) (Brachydanio rerio) |
|||||||||||||||
NCBI Taxonomy ID |
7955 |
|||||||||||||||
Chromosome Location |
|
|||||||||||||||
Function in Stage |
||||||||||||||||
Function in Cell Type |
||||||||||||||||
Description |
Temporarily unavailable |
|||||||||||||||
The information of related literatures |
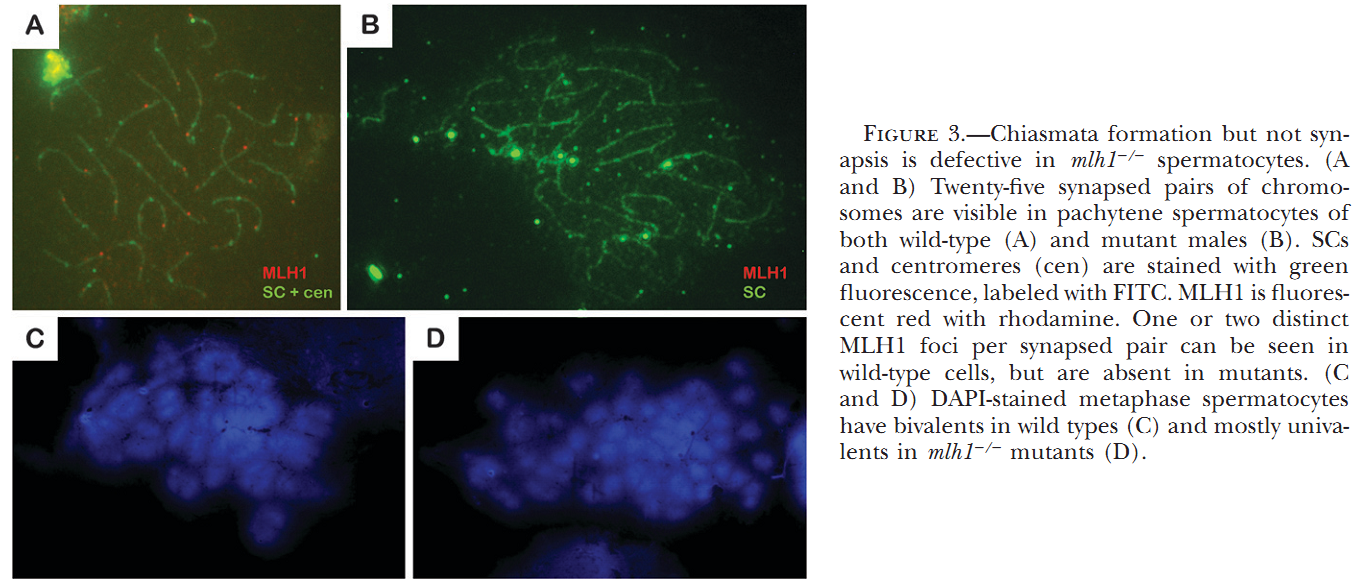
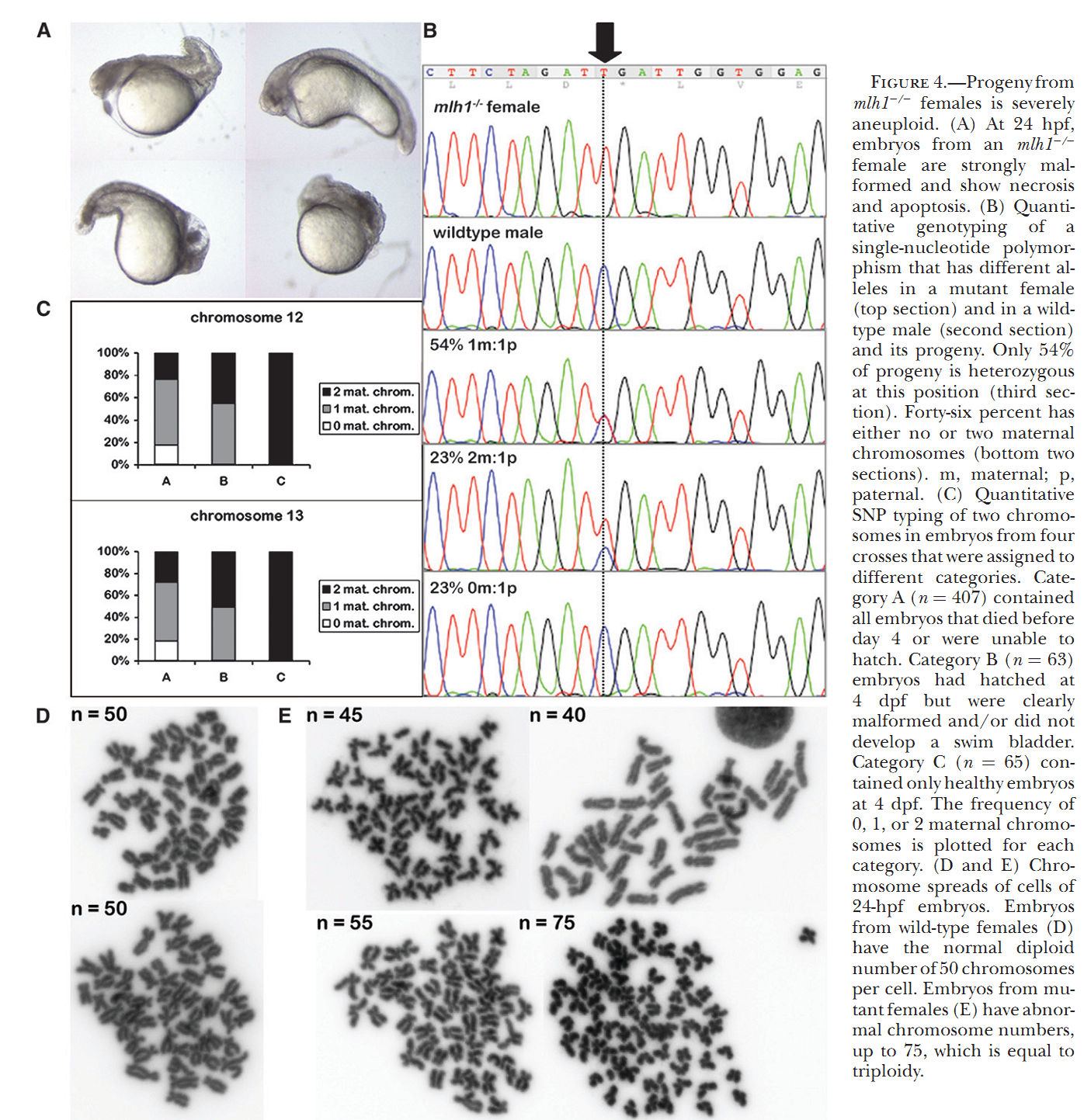
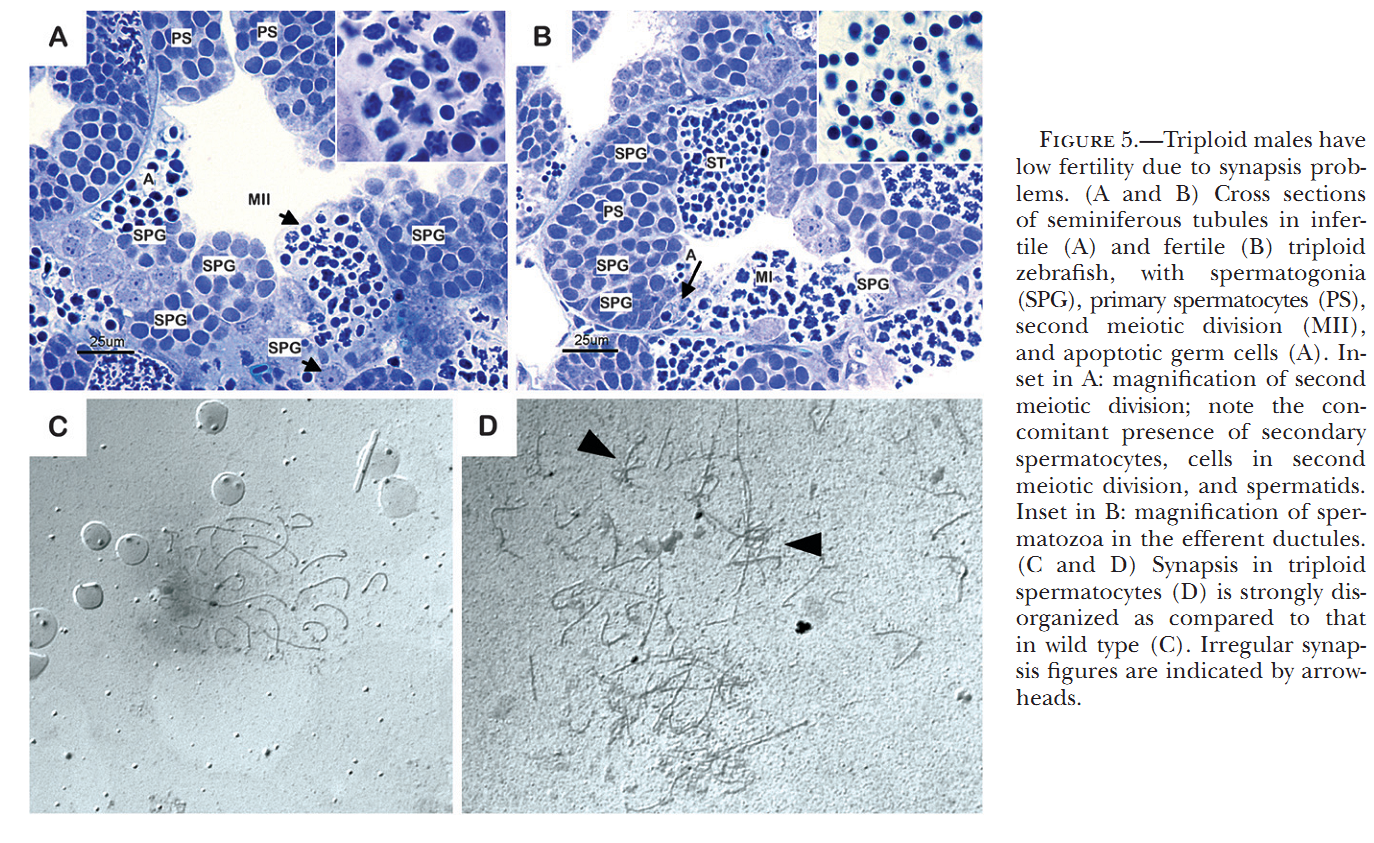
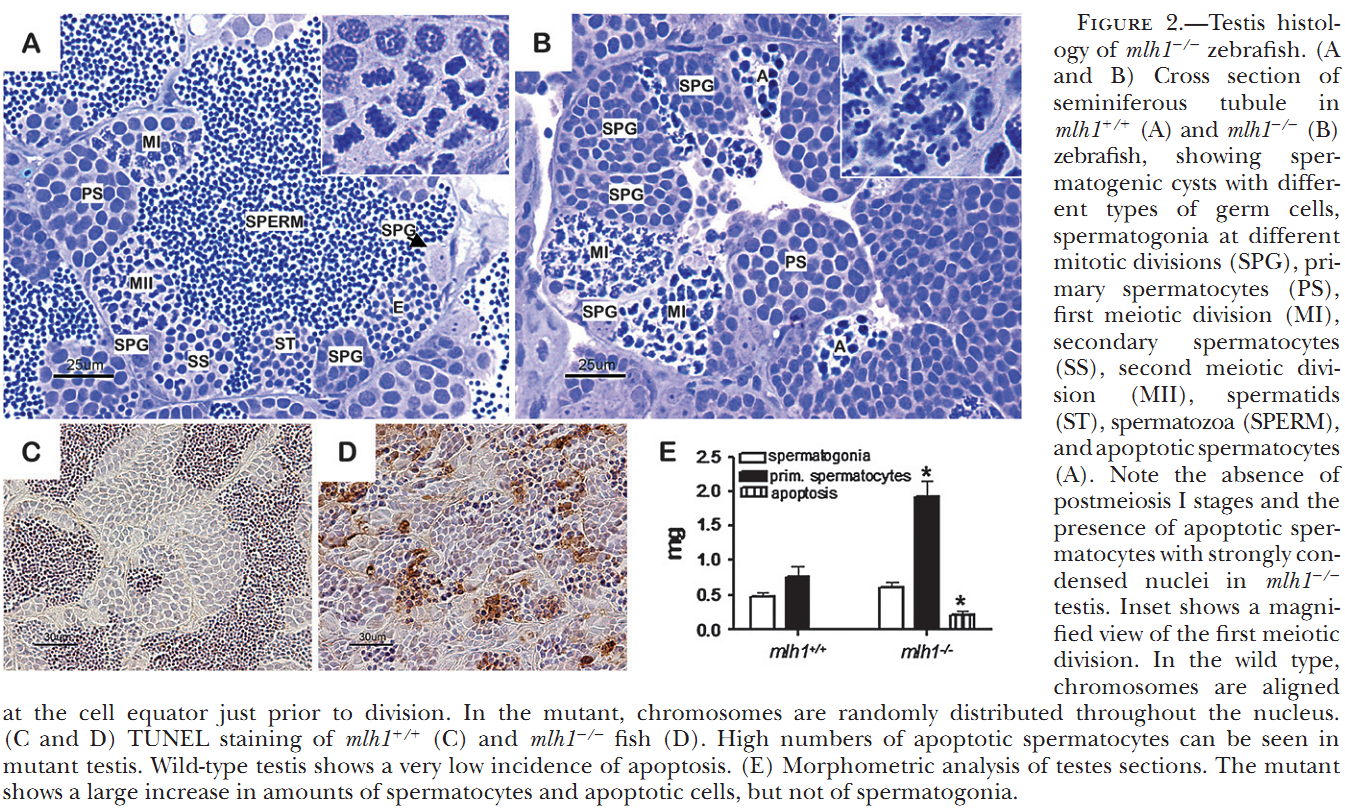
1. M. C. Leal, H. Feitsma, E. Cuppen, L. R. Franca and R. W. Schulz (2008) Completion of meiosis in male zebrafish (Danio rerio) despite lack of DNA mismatch repair gene mlh1. Cell Tissue Res 332(1): 133-9. Abstract Mlh1 is a member of DNA mismatch repair (MMR) machinery and is also essential for the stabilization of crossovers during the first meiotic division. Recently, we have shown that zebrafish mlh1 mutant males are completely infertile because of a block in metaphase I, whereas females are fertile but have aneuploid progeny. When studying fertility in males in a two-fold more inbred background, we have however observed low numbers of fertilized eggs (approximately 0.4%). Histological examination of the testis has revealed that all spermatogenic stages prior to spermatids (spermatogonia, primary spermatocytes, and secondary spermatocytes) are significantly increased in the mutant, whereas the total weight of spermatids and spermatozoa is highly decreased (1.8 mg in wild-type vs. 0.1 mg in mutants), a result clearly different from our previous study in which outbred males lack secondary spermatocytes or postmeiotic cells. Thus, a delay of both meiotic divisions occurs rather than complete arrest during meiosis I in these males. Eggs fertilized with mutant sperm develop as malformed embryos and are aneuploid making this male phenotype much more similar to that previously described in the mutant females. Therefore, crossovers are still essential for proper meiosis, but meiotic cell divisions can progress without it, suggesting that this mutant is a suitable model for studying the cellular mechanisms of completing meiosis without crossover stabilization. PMID: [18247060] 2. H. Feitsma, M. C. Leal, P. B. Moens, E. Cuppen and R. W. Schulz (2007) Mlh1 deficiency in zebrafish results in male sterility and aneuploid as well as triploid progeny in females. Genetics 175(4): 1561-9. Abstract In most eukaryotes, recombination of homologous chromosomes during meiosis is necessary for proper chromosome pairing and subsequent segregation. The molecular mechanisms of meiosis are still relatively unknown, but numerous genes are known to be involved, among which are many mismatch repair genes. One of them, mlh1, colocalizes with presumptive sites of crossing over, but its exact action remains unclear. We studied meiotic processes in a knockout line for mlh1 in zebrafish. Male mlh1 mutants are sterile and display an arrest in spermatogenesis at metaphase I, resulting in increased testis weight due to accumulation of prophase I spermatocytes. In contrast, females are fully fertile, but their progeny shows high rates of dysmorphology and mortality within the first days of development. SNP-based chromosome analysis shows that this is caused by aneuploidy, resulting from meiosis I chromosomal missegregation. Surprisingly, the small percentage of progeny that develops normally has a complete triploid genome, consisting of both sets of maternal and one set of paternal chromosomes. As adults, these triploid fish are infertile males with wild-type appearance. The frequency of triploid progeny of mlh1 mutant females is much higher than could be expected for random chromosome segregation. Together, these results show that multiple solutions exist for meiotic crossover/segregation problems. PMID: [17237513] Back to Top |
|||||||||||||||
Figures for illustrating the function of this protein/gene |
|
|||||||||||||||
Function |
||||||||||||||||
Subcellular Location |
||||||||||||||||
Tissue Specificity |
||||||||||||||||
Gene Ontology |
|
|||||||||||||||
Interpro |
||||||||||||||||
Pfam |
||||||||||||||||
SMART |
||||||||||||||||
PROSITE |
||||||||||||||||
PRINTS |
||||||||||||||||
Created Date |
18-Oct-2012 |
|||||||||||||||
Record Type |
Experiment identified |
|||||||||||||||
Protein sequence Annotation |
||||||||||||||||
Nucleotide Sequence |
Length: 158912 bp Go to nucleotide: FASTA |
|||||||||||||||
Protein Sequence |
Length: 724 bp Go to amino acid: FASTA |
|||||||||||||||
The verified Protein-Protein interaction information |
| |||||||||||||||
Other Protein-Protein interaction resources |
String database |
|||||||||||||||
View Microarray data |
Temporarily unavailable |
|||||||||||||||
Comments |
||||||||||||||||