| Tag | Content | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
SG ID |
SG00000309 |
||||||||||||||||||||||||||||||
UniProt Accession |
|||||||||||||||||||||||||||||||
Theoretical PI |
4.8
|
||||||||||||||||||||||||||||||
Molecular Weight |
14225 Da
|
||||||||||||||||||||||||||||||
Genbank Nucleotide ID |
|||||||||||||||||||||||||||||||
Genbank Protein ID |
|||||||||||||||||||||||||||||||
Gene Name |
Roc1b |
||||||||||||||||||||||||||||||
Gene Synonyms/Alias |
ROC2 |
||||||||||||||||||||||||||||||
Protein Name |
RING-box protein 1B |
||||||||||||||||||||||||||||||
Protein Synonyms/Alias |
Regulator of cullins 1b; |
||||||||||||||||||||||||||||||
Organism |
Drosophila melanogaster (Fruit fly) |
||||||||||||||||||||||||||||||
NCBI Taxonomy ID |
7227 |
||||||||||||||||||||||||||||||
Chromosome Location |
|
||||||||||||||||||||||||||||||
Function in Stage |
|||||||||||||||||||||||||||||||
Function in Cell Type |
|||||||||||||||||||||||||||||||
Description |
Temporarily unavailable |
||||||||||||||||||||||||||||||
The information of related literatures |
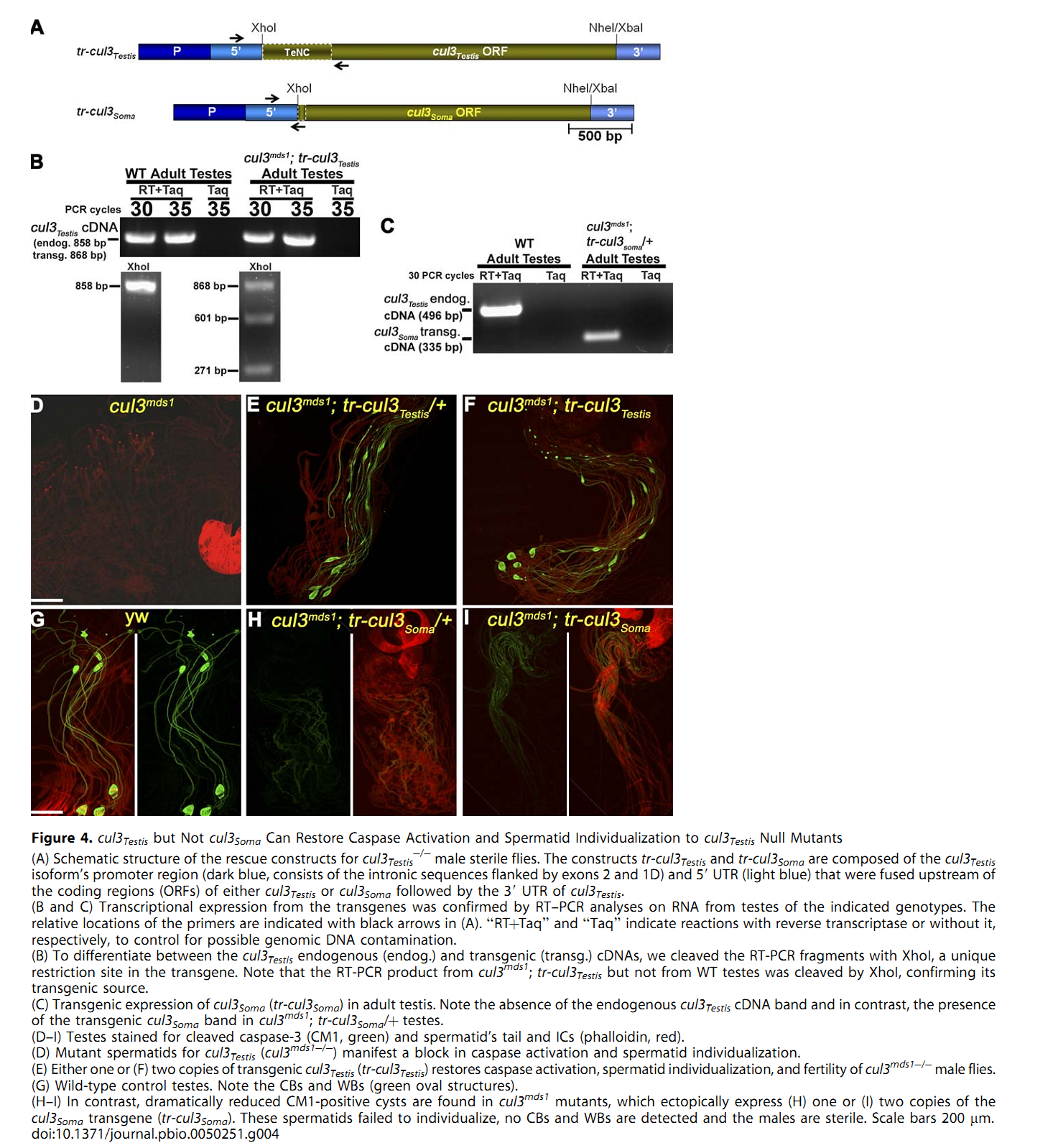
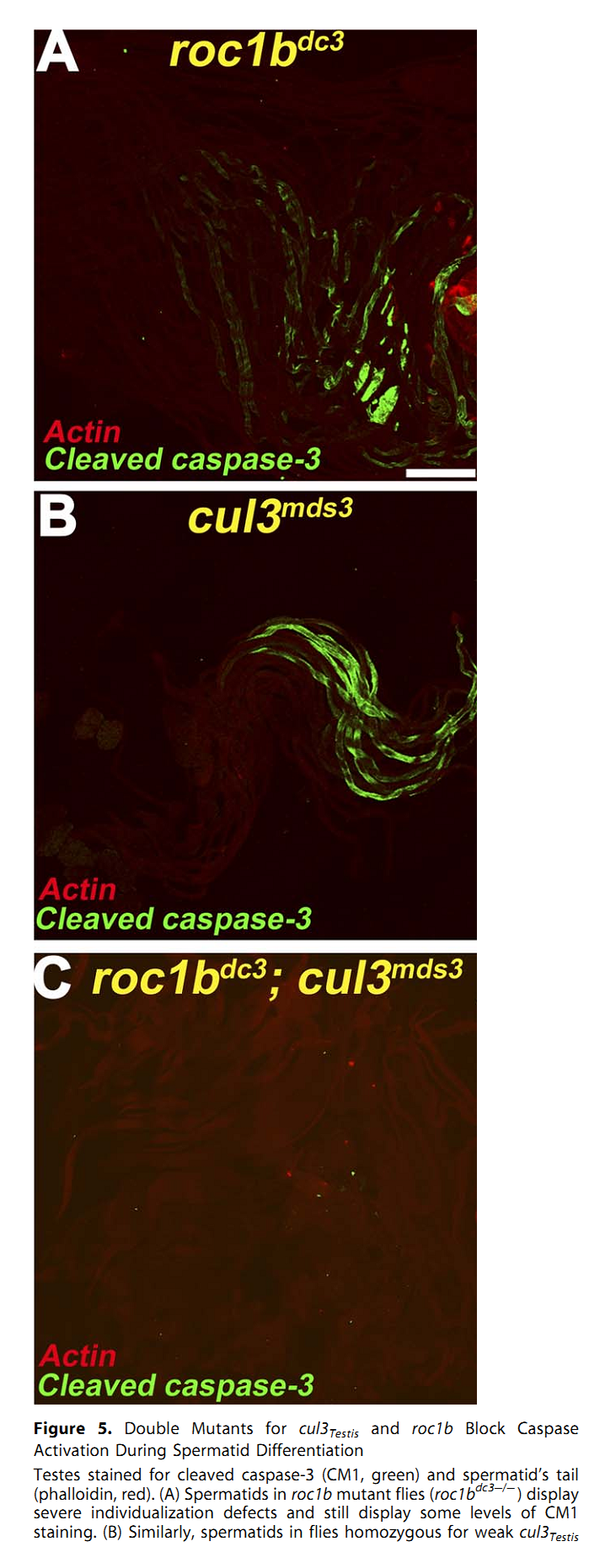
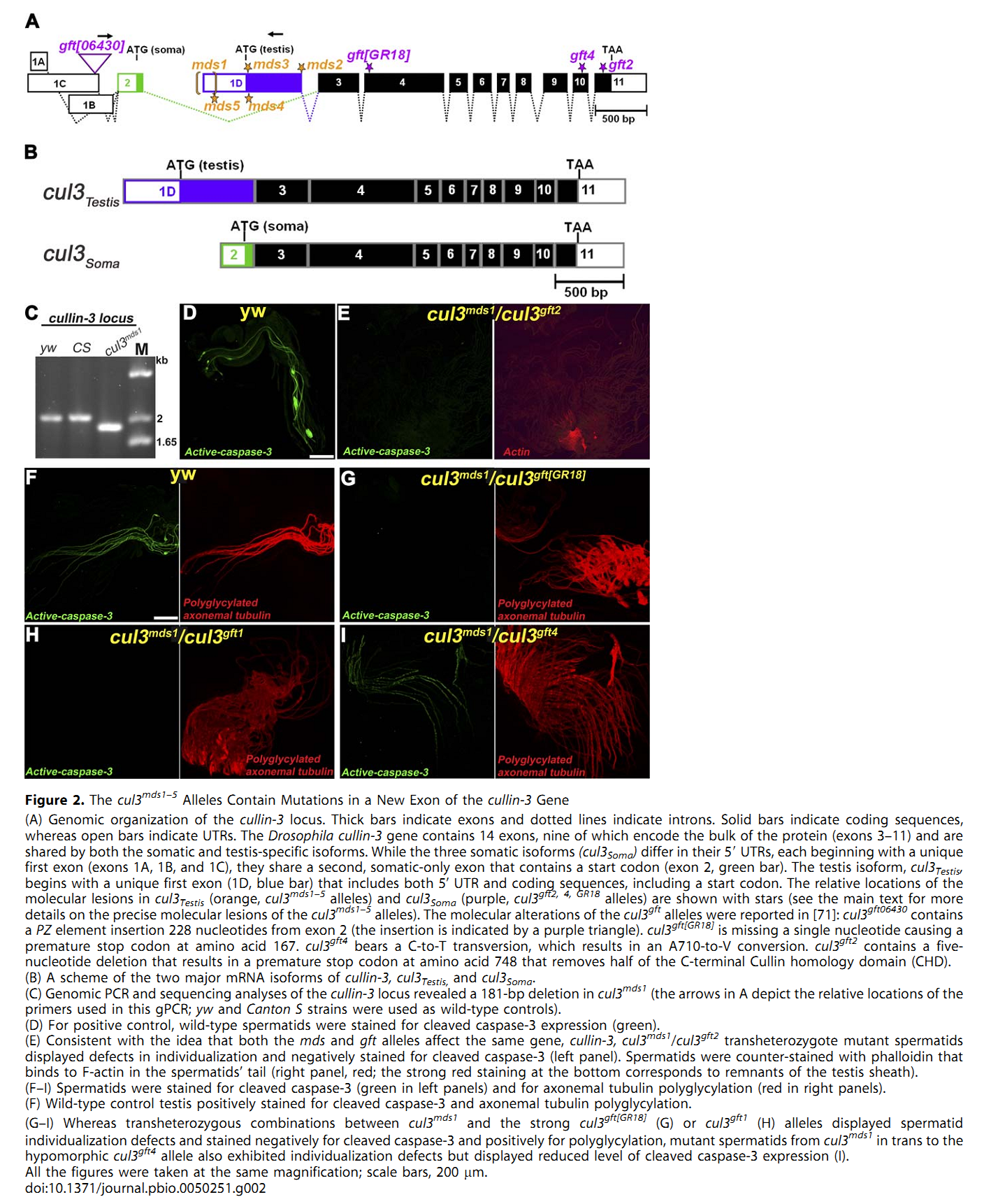
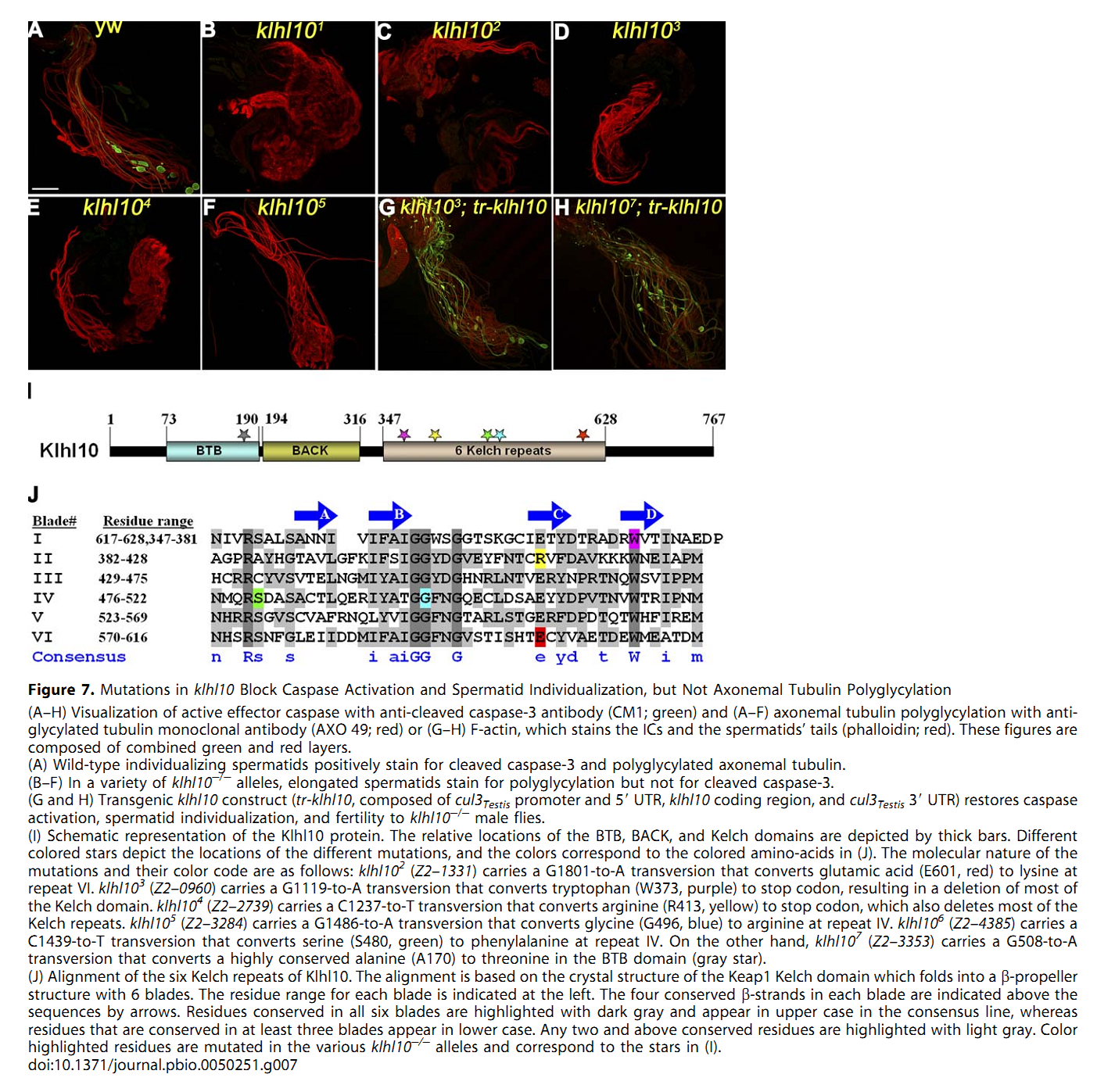
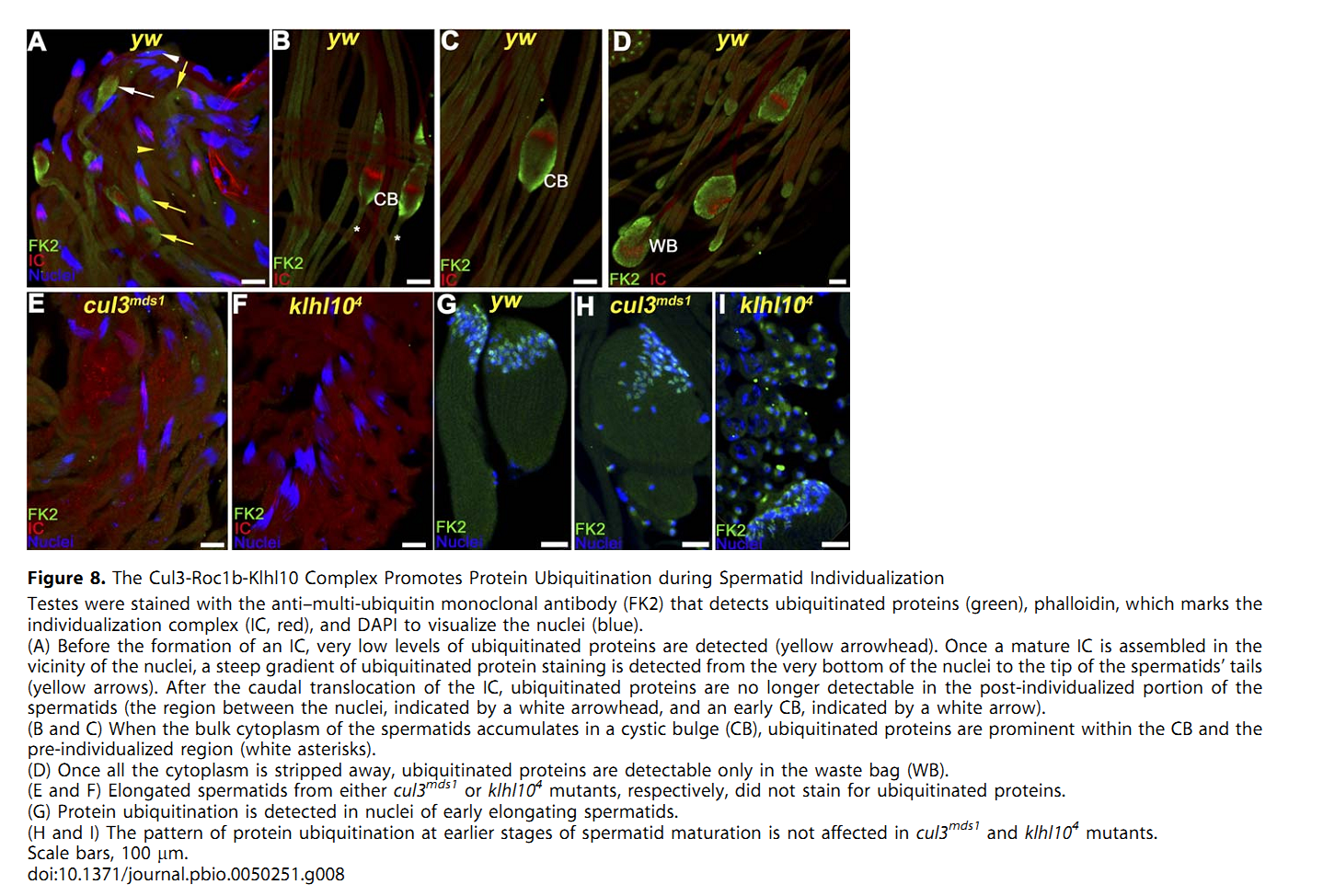
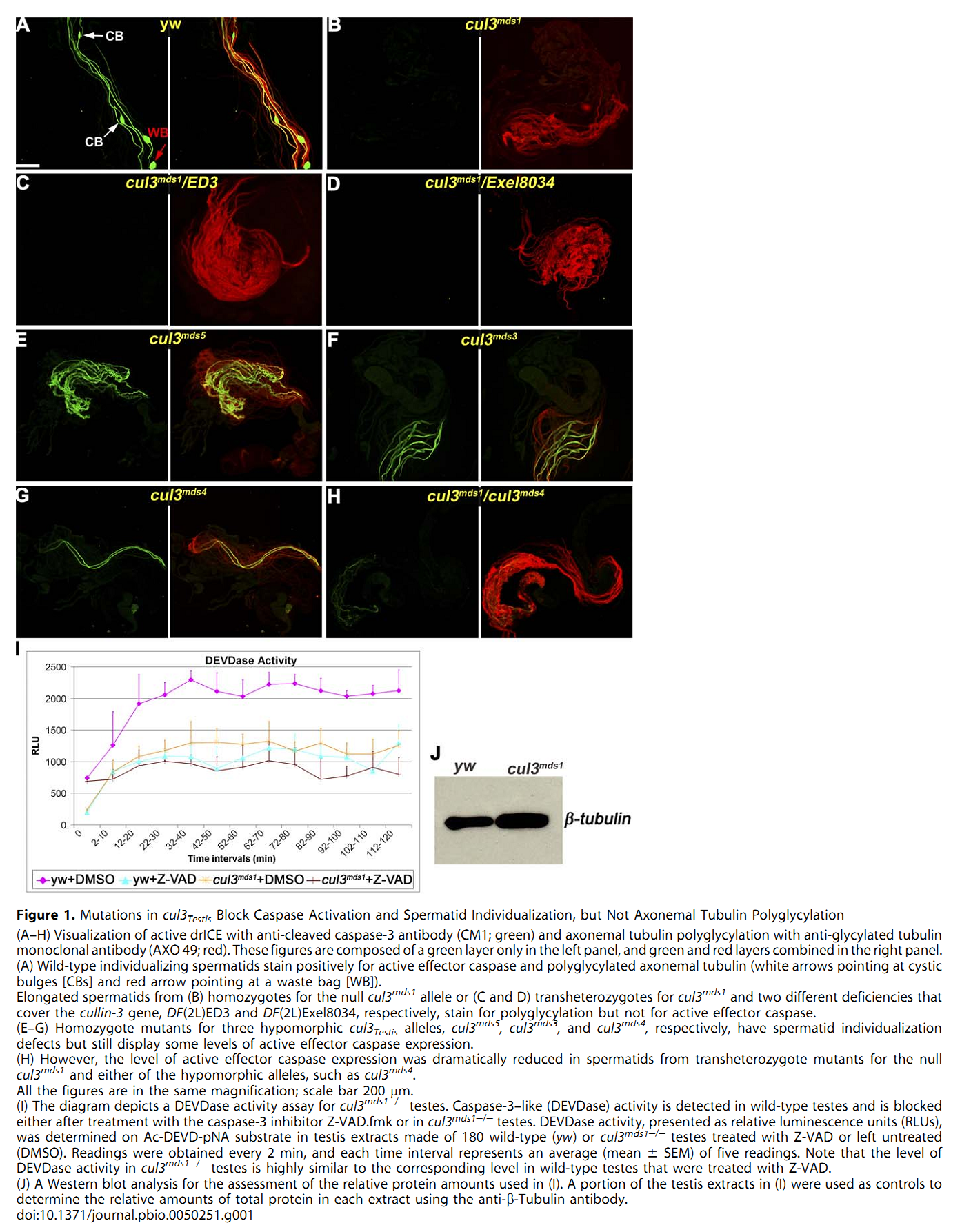
1. E. Arama, M. Bader, G. E. Rieckhof and H. Steller (2007) A ubiquitin ligase complex regulates caspase activation during sperm differentiation in Drosophila. PLoS Biol 5(10): e251. Abstract In both insects and mammals, spermatids eliminate their bulk cytoplasm as they undergo terminal differentiation. In Drosophila, this process of dramatic cellular remodeling requires apoptotic proteins, including caspases. To gain further insight into the regulation of caspases, we screened a large collection of sterile male flies for mutants that block effector caspase activation at the onset of spermatid individualization. Here, we describe the identification and characterization of a testis-specific, Cullin-3-dependent ubiquitin ligase complex that is required for caspase activation in spermatids. Mutations in either a testis-specific isoform of Cullin-3 (Cul3(Testis)), the small RING protein Roc1b, or a Drosophila orthologue of the mammalian BTB-Kelch protein Klhl10 all reduce or eliminate effector caspase activation in spermatids. Importantly, all three genes encode proteins that can physically interact to form a ubiquitin ligase complex. Roc1b binds to the catalytic core of Cullin-3, and Klhl10 binds specifically to a unique testis-specific N-terminal Cullin-3 (TeNC) domain of Cul3(Testis) that is required for activation of effector caspase in spermatids. Finally, the BIR domain region of the giant inhibitor of apoptosis-like protein dBruce is sufficient to bind to Klhl10, which is consistent with the idea that dBruce is a substrate for the Cullin-3-based E3-ligase complex. These findings reveal a novel role of Cullin-based ubiquitin ligases in caspase regulation. PMID: [17880263] Back to Top |
||||||||||||||||||||||||||||||
Figures for illustrating the function of this protein/gene |
|
||||||||||||||||||||||||||||||
Function |
Component of the SCF (SKP1-CUL1-F-box protein) E3ubiquitin ligase complex, which mediates the ubiquitination andsubsequent proteasomal degradation of target proteins. Through theRING-type zinc finger, seems to recruit the E2 ubiquitinationenzyme to the complex and brings it into close proximity to thesubstrate (By similarity). Back to Top |
||||||||||||||||||||||||||||||
Subcellular Location |
Cytoplasm. Nucleus. |
||||||||||||||||||||||||||||||
Tissue Specificity |
Highly expressed in early embryos, and inpupae. Widely expressed in adult males, while it is weaklyexpressed in adult females. |
||||||||||||||||||||||||||||||
Gene Ontology |
|
||||||||||||||||||||||||||||||
Interpro |
|||||||||||||||||||||||||||||||
Pfam |
|||||||||||||||||||||||||||||||
SMART |
|||||||||||||||||||||||||||||||
PROSITE |
|||||||||||||||||||||||||||||||
PRINTS |
|||||||||||||||||||||||||||||||
Created Date |
18-Oct-2012 |
||||||||||||||||||||||||||||||
Record Type |
Experiment identified |
||||||||||||||||||||||||||||||
Protein sequence Annotation |
CHAIN 1 122 RING-box protein 1B. /FTId=PRO_0000056018. ZN_FING 57 112 RING-type. METAL 57 57 Zinc 1 (By similarity). METAL 60 60 Zinc 1 (By similarity). METAL 68 68 Zinc 3 (By similarity). METAL 71 71 Zinc 3 (By similarity). METAL 82 82 Zinc 3 (By similarity). METAL 89 89 Zinc 2 (By similarity). METAL 91 91 Zinc 2 (By similarity). METAL 94 94 Zinc 1 (By similarity). METAL 96 96 Zinc 3 (By similarity). METAL 97 97 Zinc 1 (By similarity). METAL 108 108 Zinc 2 (By similarity). METAL 111 111 Zinc 2 (By similarity). Back to Top |
||||||||||||||||||||||||||||||
Nucleotide Sequence |
Length: 750 bp Go to nucleotide: FASTA |
||||||||||||||||||||||||||||||
Protein Sequence |
Length: 122 bp Go to amino acid: FASTA |
||||||||||||||||||||||||||||||
The verified Protein-Protein interaction information |
|||||||||||||||||||||||||||||||
Other Protein-Protein interaction resources |
String database |
||||||||||||||||||||||||||||||
View Microarray data |
Temporarily unavailable |
||||||||||||||||||||||||||||||
Comments |
|||||||||||||||||||||||||||||||