| Tag | Content | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
SG ID |
SG00000321 |
||||||||||||||||||
UniProt Accession |
|||||||||||||||||||
Theoretical PI |
5.26
|
||||||||||||||||||
Molecular Weight |
76267 Da
|
||||||||||||||||||
Genbank Nucleotide ID |
|||||||||||||||||||
Genbank Protein ID |
|||||||||||||||||||
Gene Name |
yuri |
||||||||||||||||||
Gene Synonyms/Alias |
ORFNames=Dmel_CG31732 |
||||||||||||||||||
Protein Name |
|||||||||||||||||||
Protein Synonyms/Alias |
SubName: Yuri gagarin, isoform F |
||||||||||||||||||
Organism |
Drosophila melanogaster (Fruit fly) |
||||||||||||||||||
NCBI Taxonomy ID |
7227 |
||||||||||||||||||
Chromosome Location |
|
||||||||||||||||||
Function in Stage |
|||||||||||||||||||
Function in Cell Type |
|||||||||||||||||||
Description |
Temporarily unavailable |
||||||||||||||||||
The information of related literatures |
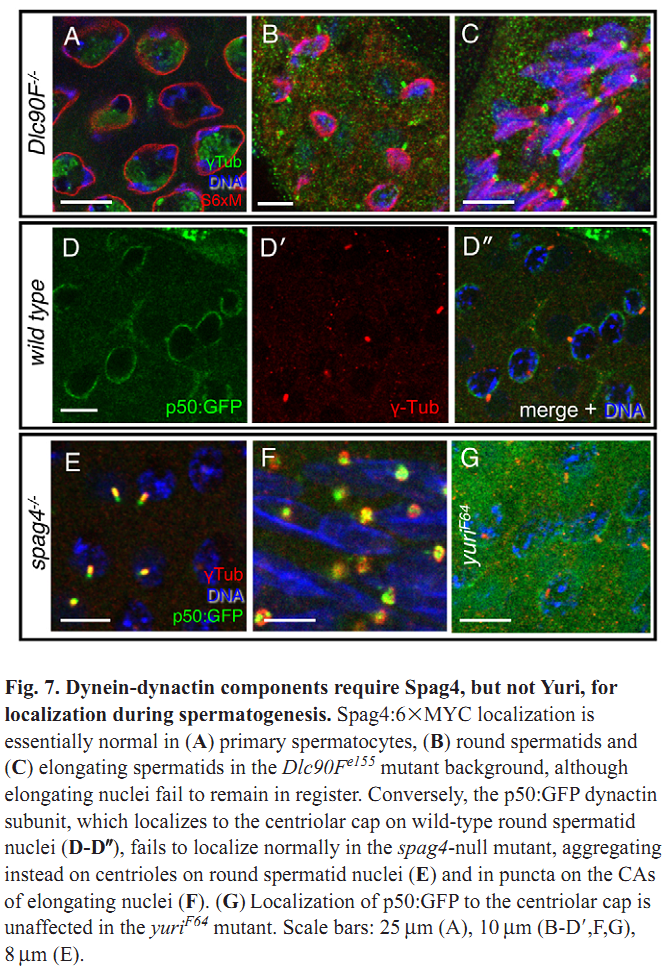
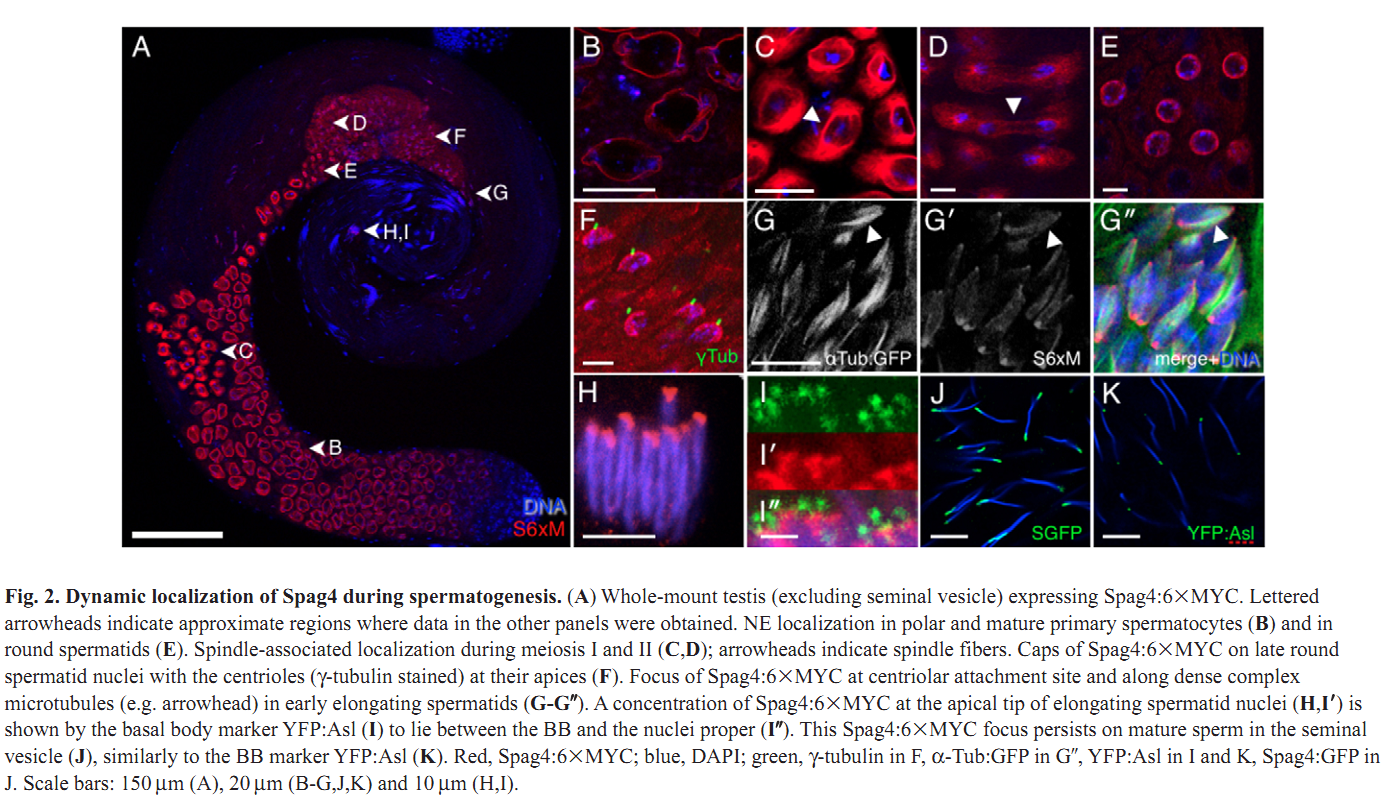
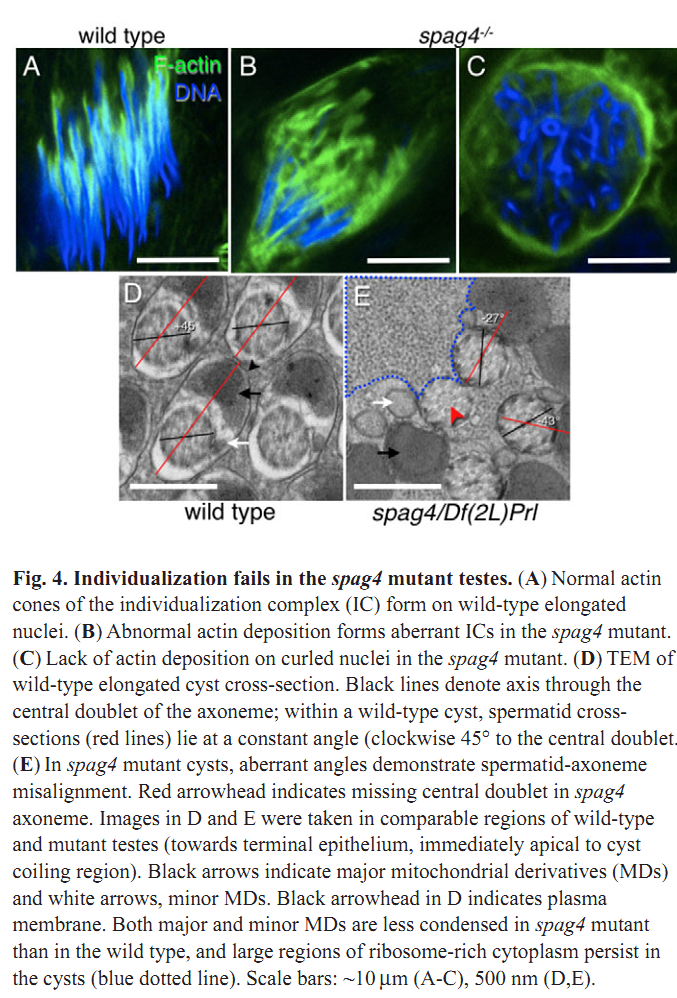
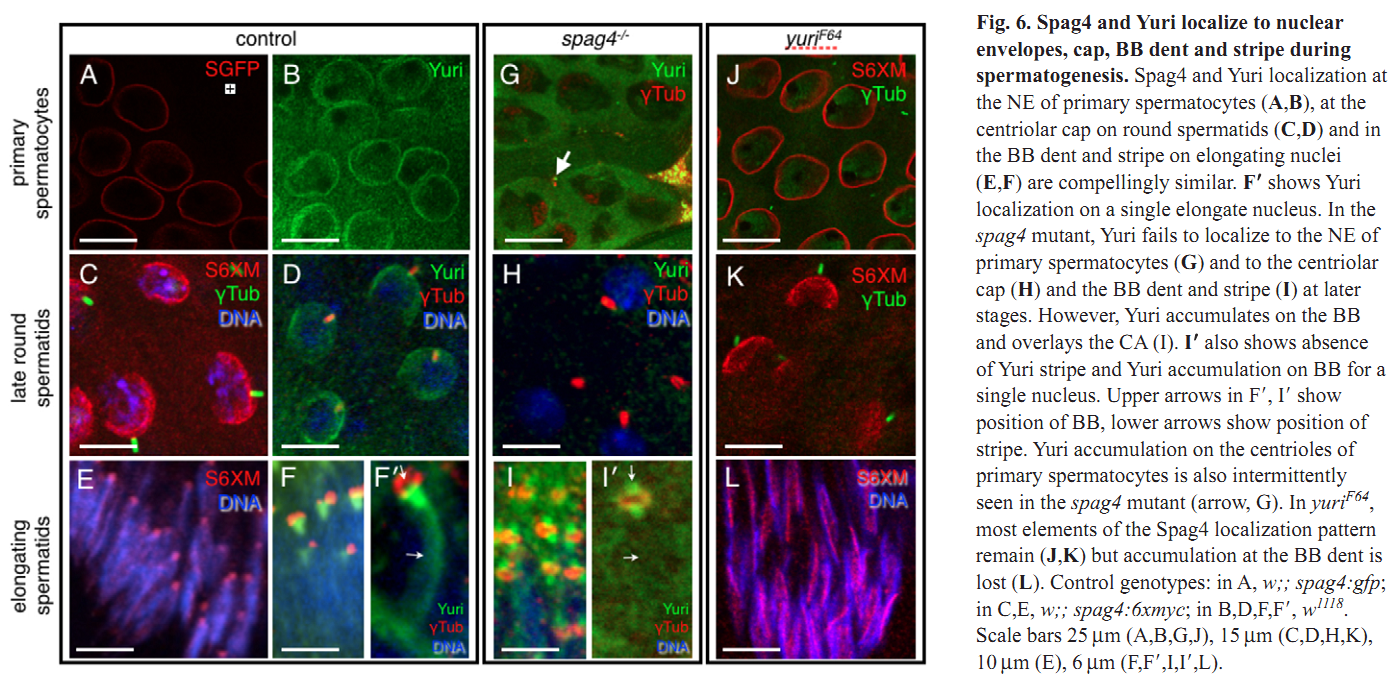
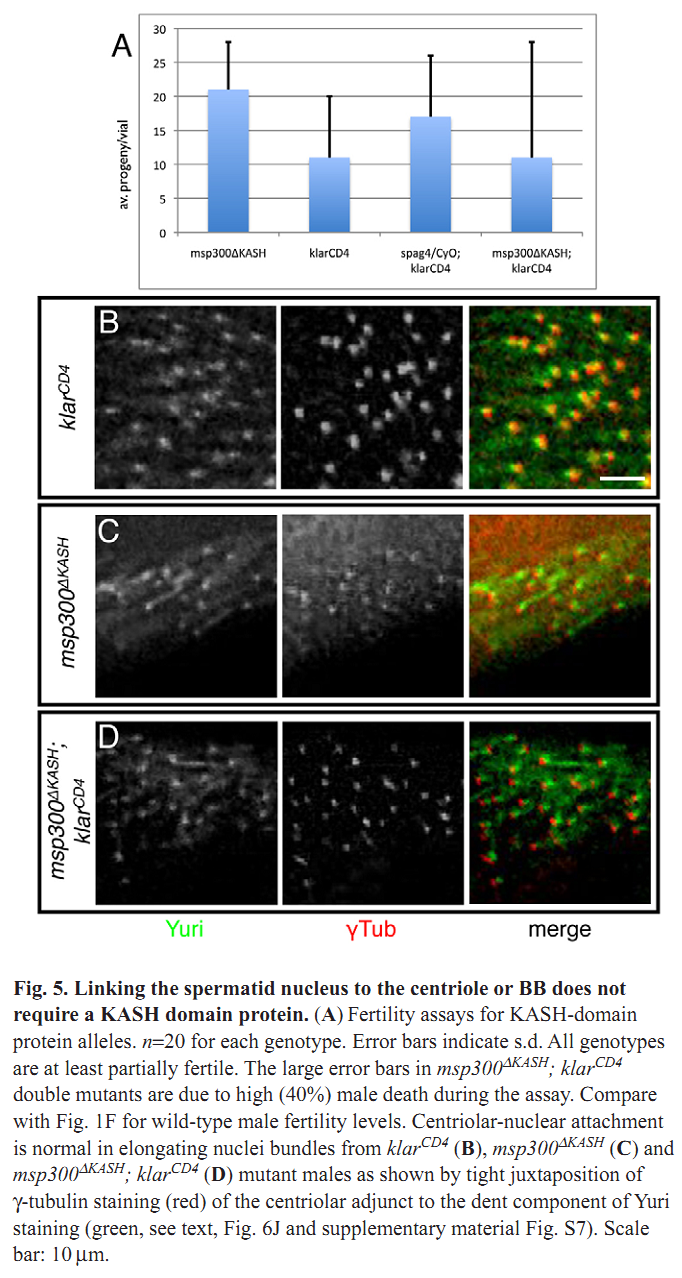
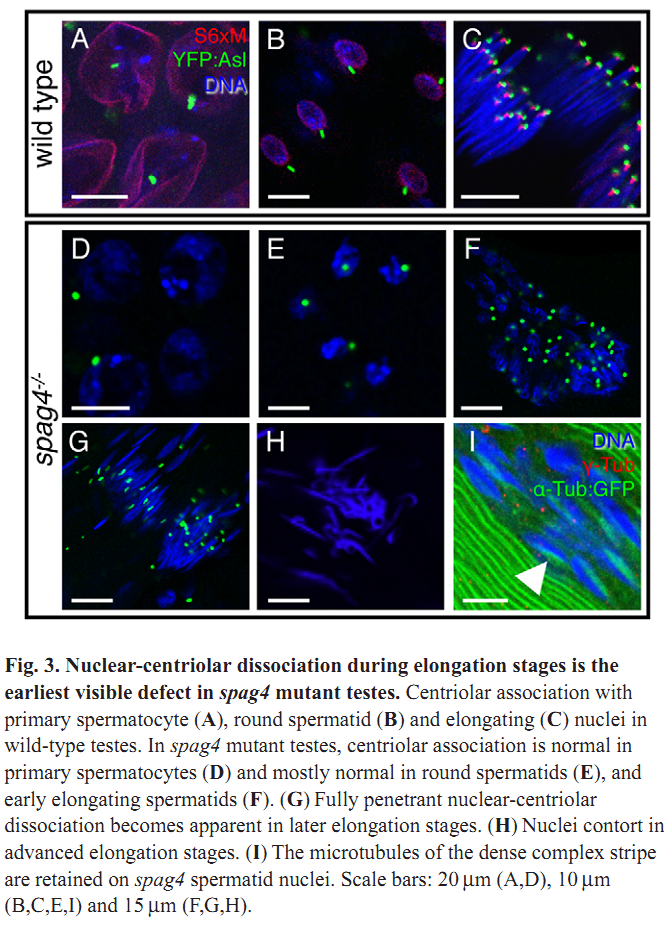
1. M. J. Texada, R. A. Simonette, C. B. Johnson, W. J. Deery and K. M. Beckingham (2008) Yuri gagarin is required for actin, tubulin and basal body functions in Drosophila spermatogenesis. J Cell Sci 121(Pt 11): 1926-36. Abstract Males of the genus Drosophila produce sperm of remarkable length. Investigation of giant sperm production in Drosophila melanogaster has demonstrated that specialized actin and microtubule structures play key roles. The gene yuri gagarin (yuri) encodes a novel protein previously identified through its role in gravitaxis. A male-sterile mutation of yuri has revealed roles for Yuri in the functions of the actin and tubulin structures of spermatogenesis. Yuri is a component of the motile actin cones that individualize the spermatids and is essential for their formation. Furthermore, Yuri is required for actin accumulation in the dense complex, a microtubule-rich structure on the sperm nuclei thought to strengthen the nuclei during elongation. In the yuri mutant, late clusters of syncytial nuclei are deformed and disorganized. The basal bodies are also mispositioned on the nuclei, and the association of a specialized structure, the centriolar adjunct (CA), with the basal body is lost. Some of these nuclear defects might underlie a further unexpected abnormality PMID: [18477609] 2. M. P. Kracklauer, H. M. Wiora, W. J. Deery, X. Chen, B. Bolival, Jr., D. Romanowicz, R. A. Simonette, M. T. Fuller, J. A. Fischer and K. M. Beckingham (2010) The Drosophila SUN protein Spag4 cooperates with the coiled-coil protein Yuri Gagarin to maintain association of the basal body and spermatid nucleus. J Cell Sci 123(Pt 16): 2763-72. Abstract Maintaining the proximity of centrosomes to nuclei is important in several cellular contexts, and LINC complexes formed by SUN and KASH proteins are crucial in this process. Here, we characterize the presumed Drosophila ortholog of the mammalian SUN protein, sperm-associated antigen 4 (Spag4, previously named Giacomo), and demonstrate that Spag4 is required for centriole and nuclear attachment during spermatogenesis. Production of spag4 mRNA is limited to the testis, and Spag4 protein shows a dynamic pattern of association with the germline nuclei, including a concentration of protein at the site of attachment of the single spermatid centriole. In the absence of Spag4, nuclei and centrioles or basal bodies (BBs) dissociate from each other after meiosis. This role of Spag4 in centriolar attachment does not involve either of the two KASH proteins of the Drosophila genome (Klarsicht and MSP-300), but does require the coiled-coil protein Yuri Gagarin. Yuri shows an identical pattern of localization at the nuclear surface to Spag4 during spermatogenesis, and epistasis studies show that the activities of Yuri and dynein-dynactin are downstream of spag4 in this centriole attachment pathway. The later defects in spermatogenesis seen for yuri and spag4 mutants are similar, suggesting they could be secondary to initial disruption of events at the nuclear surface. PMID: [20647369] Back to Top |
||||||||||||||||||
Figures for illustrating the function of this protein/gene |
|
||||||||||||||||||
Function |
|||||||||||||||||||
Subcellular Location |
|||||||||||||||||||
Tissue Specificity |
|||||||||||||||||||
Gene Ontology |
|
||||||||||||||||||
Interpro |
|||||||||||||||||||
Pfam |
|||||||||||||||||||
SMART |
|||||||||||||||||||
PROSITE |
|||||||||||||||||||
PRINTS |
|||||||||||||||||||
Created Date |
18-Oct-2012 |
||||||||||||||||||
Record Type |
Experiment identified |
||||||||||||||||||
Protein sequence Annotation |
|||||||||||||||||||
Nucleotide Sequence |
Length: bp Go to nucleotide: FASTA |
||||||||||||||||||
Protein Sequence |
Length: 660 bp Go to amino acid: FASTA |
||||||||||||||||||
The verified Protein-Protein interaction information |
|||||||||||||||||||
Other Protein-Protein interaction resources |
String database |
||||||||||||||||||
View Microarray data |
Temporarily unavailable |
||||||||||||||||||
Comments |
|||||||||||||||||||