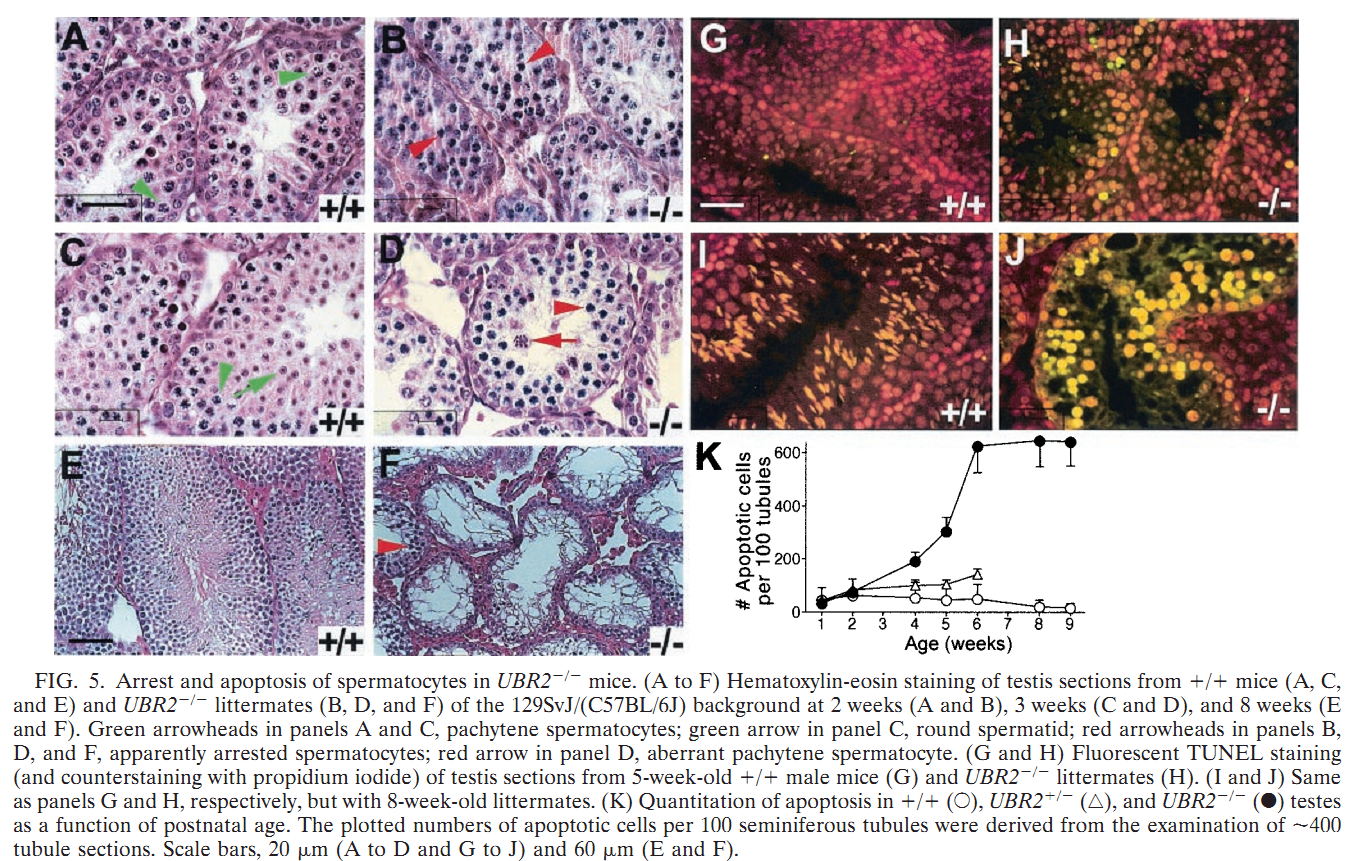
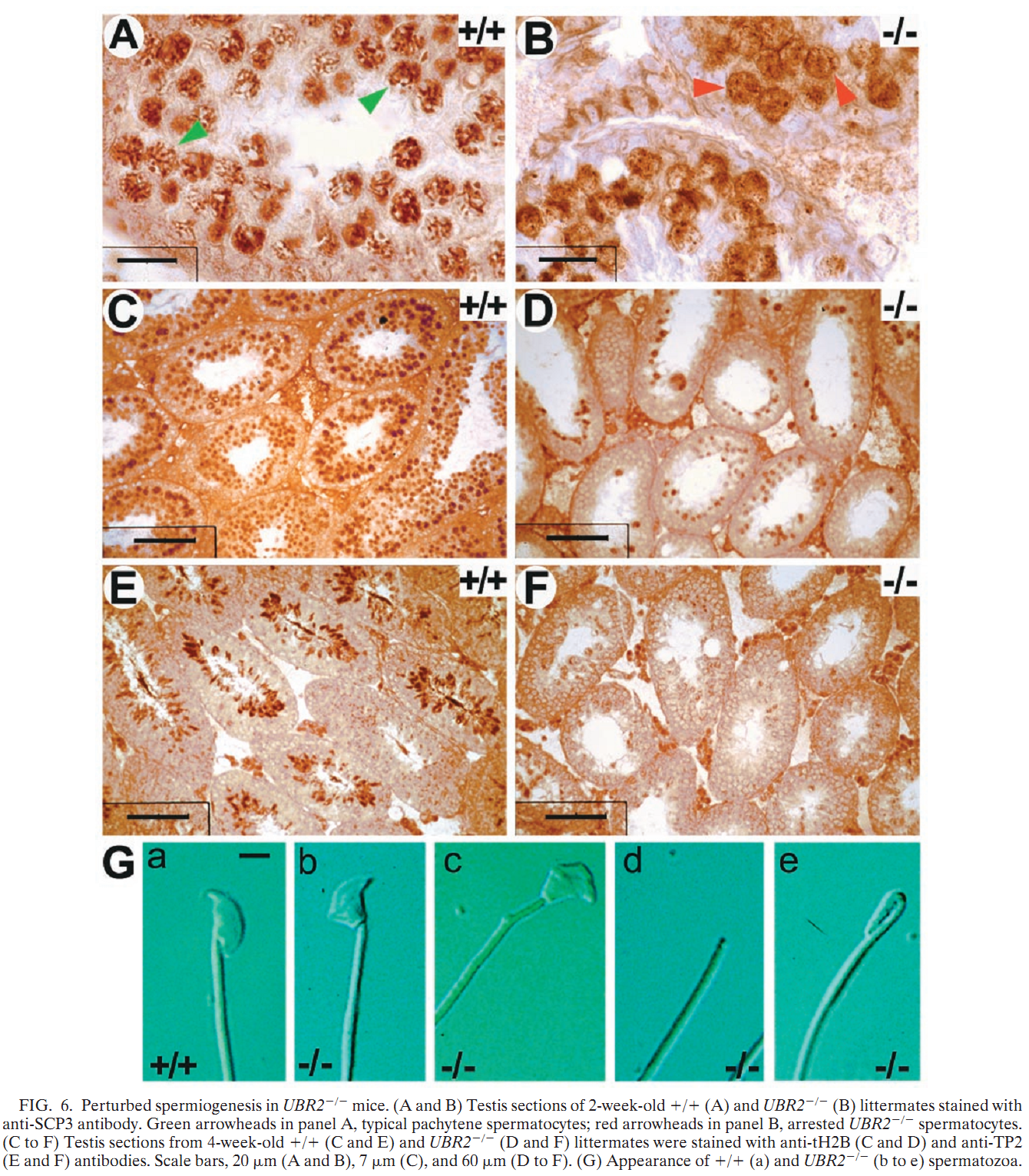
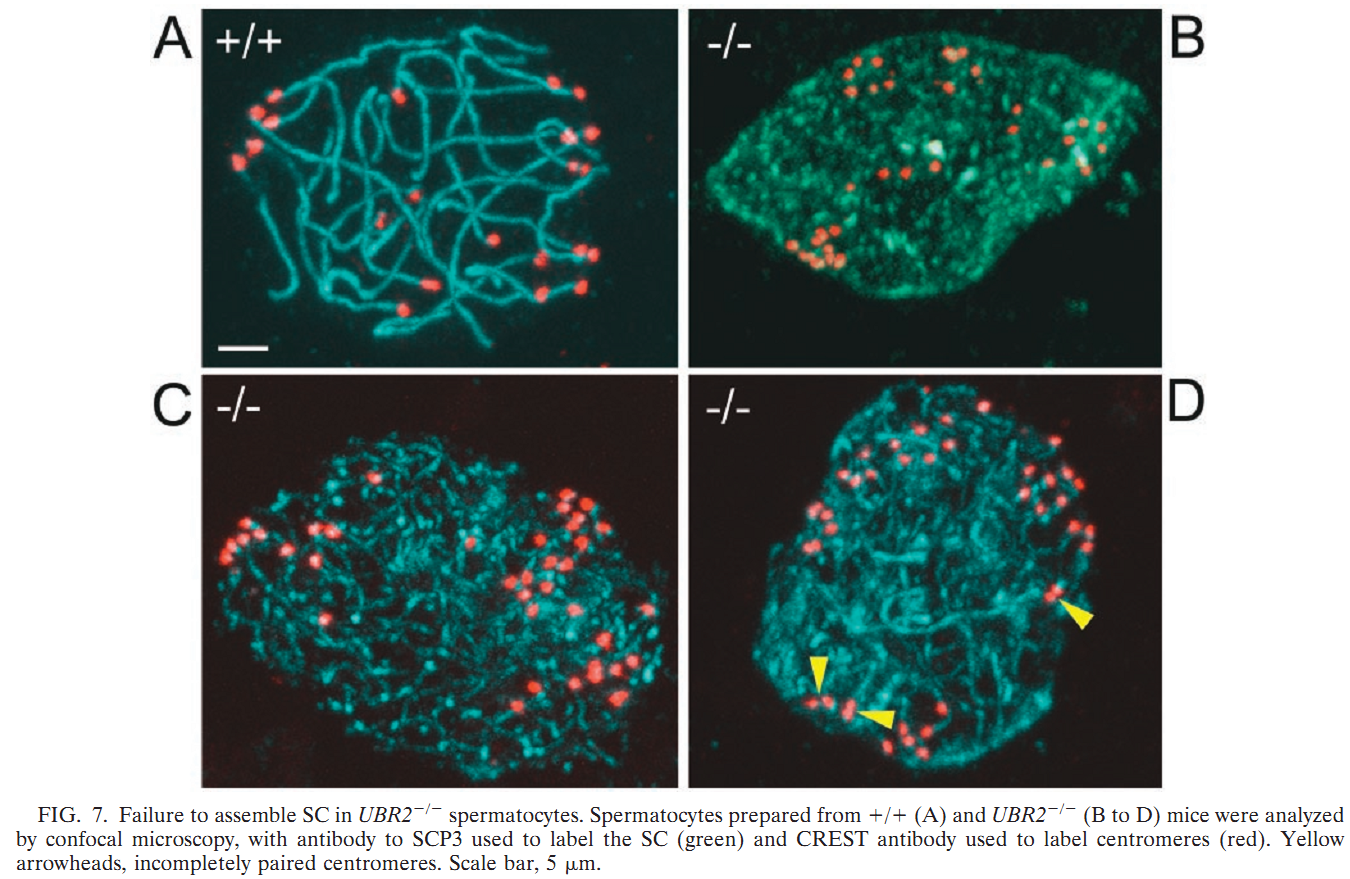
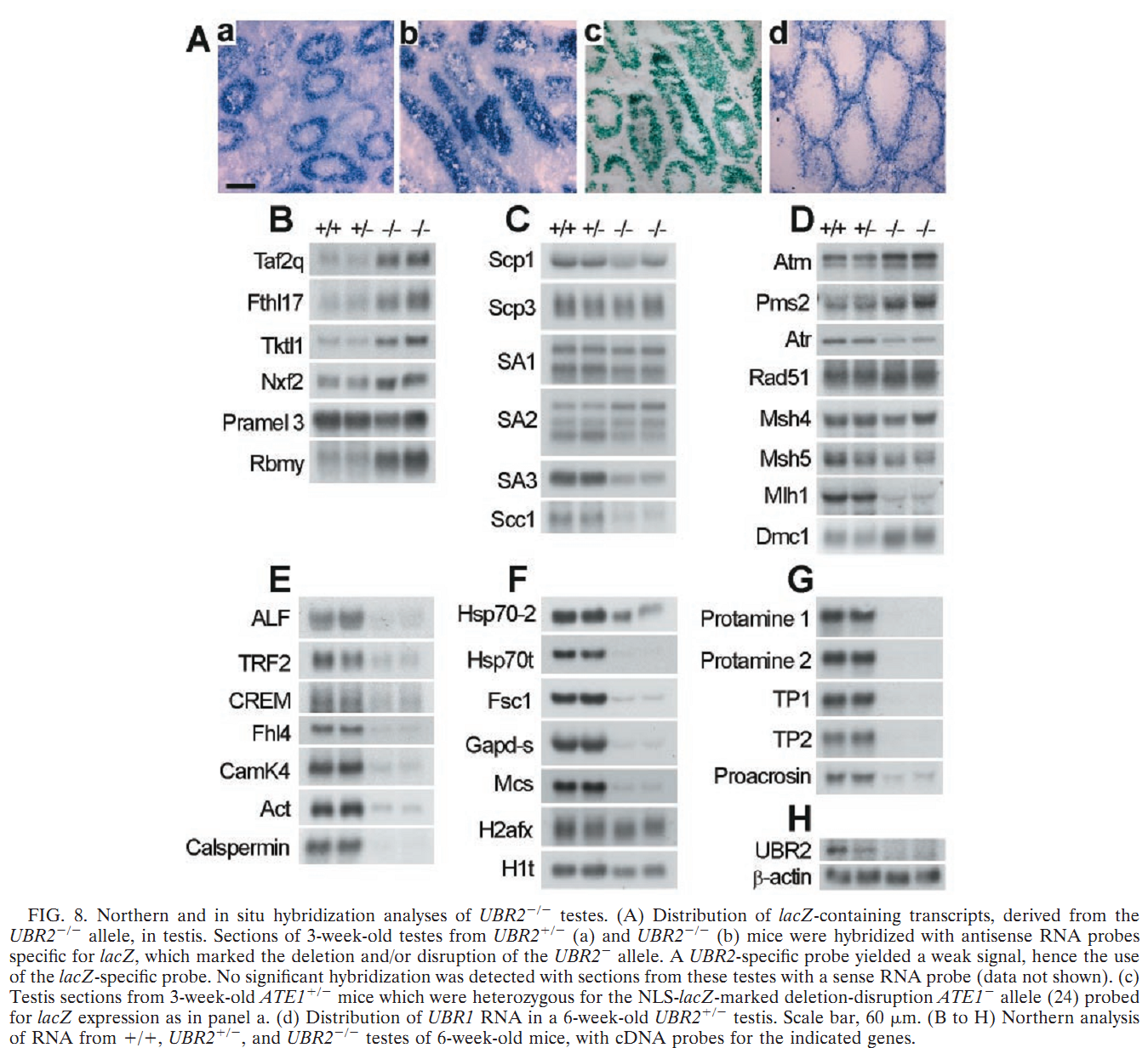
General information
| SG001367 | |||||||||||||||||
| Ubr2 | |||||||||||||||||
| 9930021A08Rik, AI462103, AW540746, E130209G04Rik, mKIAA0349 | |||||||||||||||||
| Mus musculus | |||||||||||||||||
| 10090 | |||||||||||||||||
| ENSMUSG00000023977 | |||||||||||||||||
| ENSMUSP00000108963 ENSMUSP00000108961 | |||||||||||||||||
Ubiquitin protein ligase E3 component n-recognin 2 [Mus musculus (house mouse)] | |||||||||||||||||
| |||||||||||||||||
Reviewed functional gene |
Functional information
| meiotic | |
| Spermatocyte | |
|
1. Abstract 2. Abstract 3. Abstract 4. Abstract | |
| |
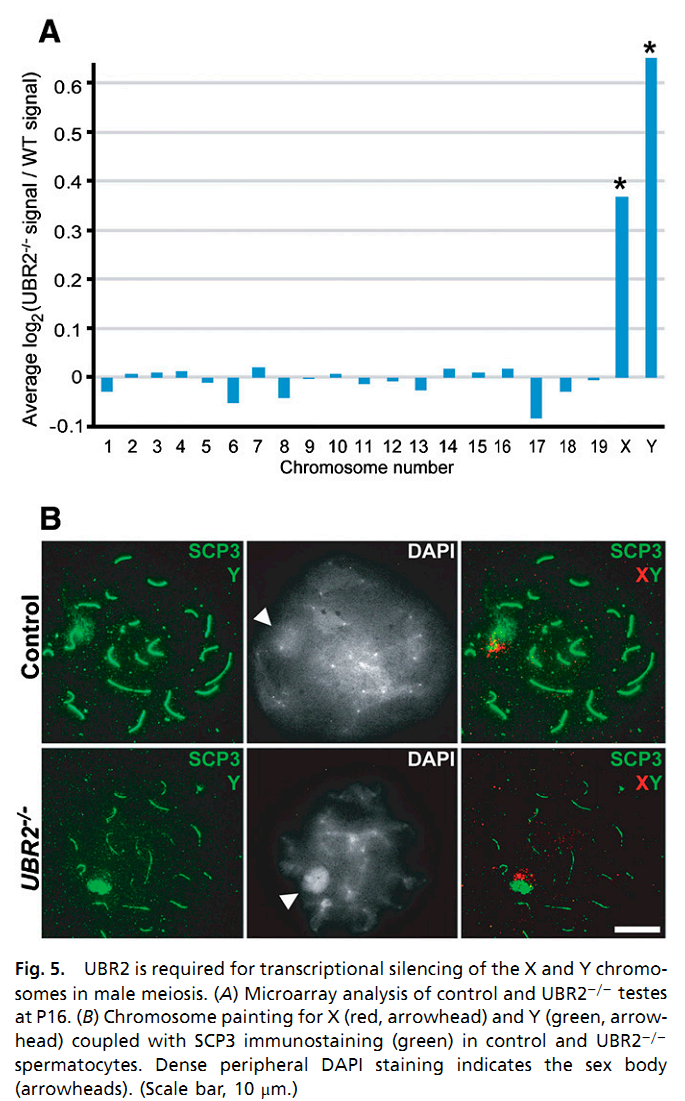
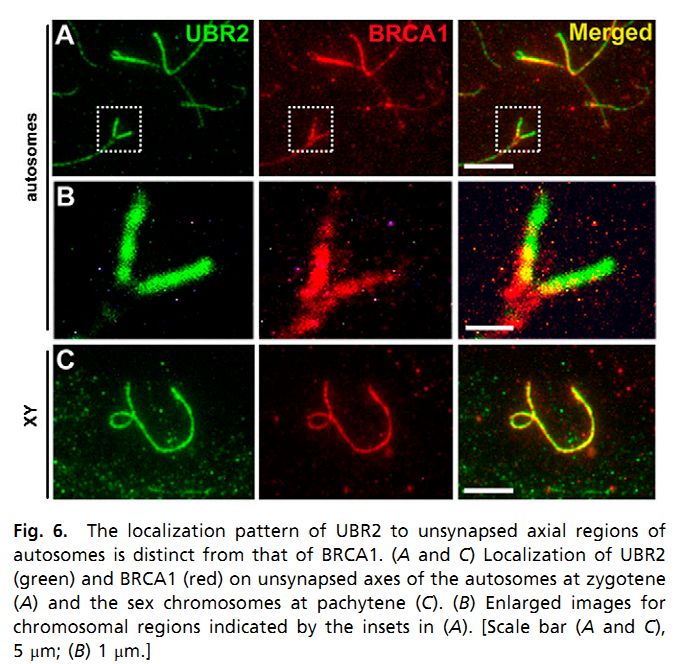
| The ubiquitin ligase Ubr2, a recognition E3 component of the N-end rule proteolytic pathway, recognizes proteins with N-terminal destabilizing residues. The UBR2 ubiquitin ligase and, hence, the N-end rule pathway are required for male meiosis and spermatogenesis and for an essential aspect of female embryonic development. The ubiquitin ligase Ubr2 maintains genome integrity and invovles in homologous recombination repair. | |
|
ReactomeID: R-MMU-983168 Antigen processing: Ubiquitination & Proteasome degradation | |
| N/A |
Expression and location
| Expressed highest in thyroid gland | |
| Expressed highest in spermatogonium | |
| View detail | |
| View detail | |
| Nucleus |
Mutation in human orthology
|
1000G (Phase 3): 3414 ESP6500 (SI-V2): 338 ExAC (r0.3.1): 1694 dbSNP(Build 147): 8360 |
|||||||
|
Chinese health control (254): 21 European health control (283): 11 Chinese patients (168): |
|||||||
|
Other information
|
Click the right sign for more information
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Q6WKZ8 |