Spermatogenesis, also known as male germ cell development, is essential for the production of sperm and male fertility. During spermatogenesis, sperm are produced from spermatogonial stem cells (SSC) sequentially through mitosis of spermatogonia, meiosis of spermatocytes, and spermiogenesis. Being a complex process, number of basic biological events are involved, such as stem cell renewal and differentiation of the SSCs, epigenetic modification and DNA genome reorganization in meiotic cells, and genome repackaging in the spermatids (1-4). Additionally, cellular interactions and communications between the germ cells and somatic components such as Sertoli and Leydig cells are also crucial for normal spermatogenesis and contribute to its complexity (5,6).
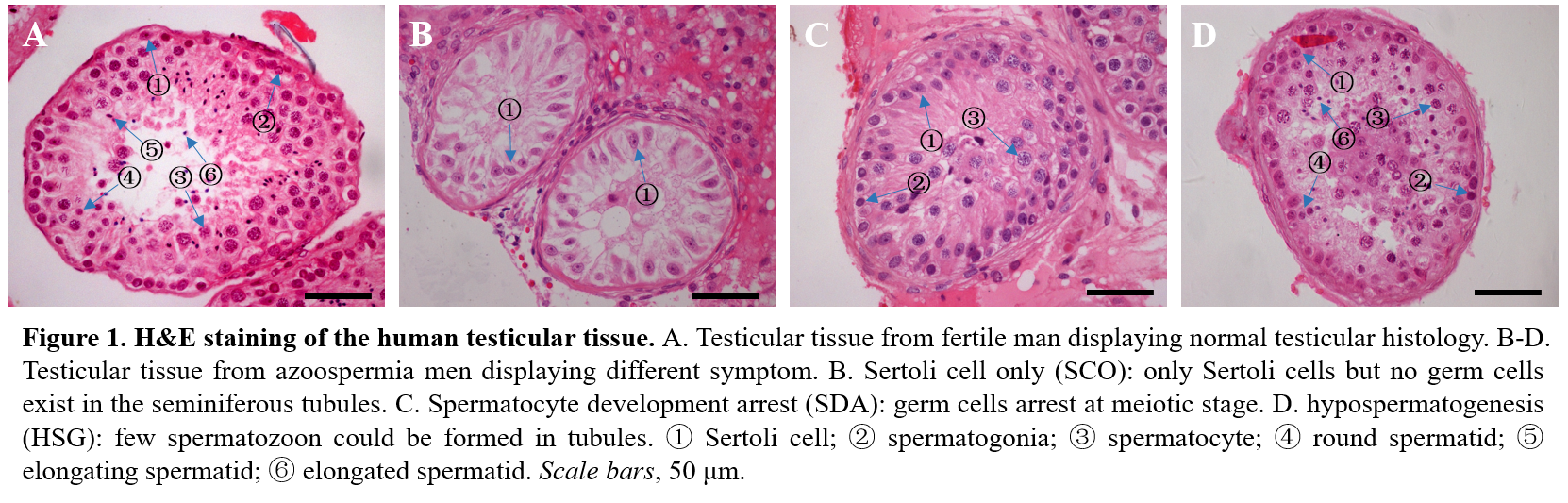
In human, infertility affects 10-15% of couples, half of which is attributed to the male partner (7) and dysregulation of spermatogenesis at any step can lead to serious reproductive problems. For example, abnormal SSC proliferation may lead to Sertoli cell only (SCO) syndrome, abnormalities at meiotic stage will result in spermatocyte development arrest (SDA), and hypospematogenesis (HSG) always attributed from abnormal spermatid processing (8-10) (Figure 1). It has been estimated that the male germ cell transcriptome comprises more than 24,000 transcripts (11), theoretically, defects in any one of these genes could lead to infertility. However, to date, only a few of genes mutation have been validated in infertile men, for example, with mutations in only two genes, SPO11 and SYCP3, reported to be associated with human SDA (12,13). Thus, the genetic basis leading to human infertility are largely unknown and this limited knowledge of the genetic causes about human infertility, resulted in very few treatment or targeted therapeutic options.
Although the ideal approach to identify human infertility causing mutations is to conduct studies in the human directly, this approach has progressed slowly due to the limitations as the complex human spermatogenesis cannot be modeled in vitro. Thanks to the similar testicular structure and comparable male germ cell development process between human and animals, the substantial acceleration in the identification and characterization of genes associated with animal spermatogenesis can provide us with the best means to reveal infertility-associated genes in human spermatogenesis (10,14).
Here, to better understand the genetic basis of human spermatogenesis and infertility, we have constructed FertilityOnline, which integrates the functional spermatogenic genes reported in literature and also incorporates the only existing functional spermatogenic database, SpermatogenesisOnline 1.0 In FertilityOnline (https://mcg.ustc.edu.cn/bsc/spermgenes2.0/), besides manually curated functional annotations such as protein complex and pathway, human diseases and detailed phenotype of spermatogenesis failure, more new features were introduced as 1) 483 newly identified functional genes were manually curated from the literature (totally 1879 genes from 42 organisms); 1) extended the organism from 37 to 42 with 483 newly identified functional genes collected; 2) mapping all the reported functional genes to human counterpart with orthology annotation; 3) adding more manual curated functional annotations such as protein complex and pathways, human diseases and detailed phenotype of spermatogenesis failure; 4) refined expression information with provided RNA-seq dataset which detect the gene expression in different human tissue and testicular cell type; 5) provided genes mutation information including statistical results of variants founded in public database, variants founded in our in-house dataset (537 healthy control with reproductive history and 168 male infertile patients) and De novo mutation rates; 6) updated the search and browse function to provided filter and download function; 7) created an analysis module which allow user to upload their own variants or gene list to our server, and Fertility Online annotated the list with all available information in it. Furthermore, user can filter the list to prioritize the candidate gene causing infertility base on the annotations via the web page and perform enrichment analysis directly. As a case study, we successfully find two candidate gene in two sporadic male infertile patients. In conclusion, Fertility Online can, not only be an updated resource for aggregation spermatogenesis but also be a useful tools for prioritize candidate human infertile variants or genes.
References
1. Handel, M.A. and Schimenti, J.C. (2010) Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nature reviews. Genetics, 11, 124-136.
2. Brinster, R.L. (2007) Male germline stem cells: from mice to men. Science, 316, 404-405.
3. Govin, J., Caron, C., Lestrat, C., Rousseaux, S. and Khochbin, S. (2004) The role of histones in chromatin remodelling during mammalian spermiogenesis. European journal of biochemistry, 271, 3459-3469.
4. Krausz, C., Escamilla, A.R. and Chianese, C. (2015) Genetics of male infertility: from research to clinic. Reproduction, 150, R159-174.
5. Mruk, D.D. and Cheng, C.Y. (2004) Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocrine reviews, 25, 747-806.
6. Wen, Q., Cheng, C.Y. and Liu, Y.X. (2016) Development, function and fate of fetal Leydig cells. Seminars in cell & developmental biology, 59, 89-98.
7. De Kretser, D.M. and Baker, H.W. (1999) Infertility in men: recent advances and continuing controversies. The Journal of clinical endocrinology and metabolism, 84, 3443-3450.
8. Ohta, H., Tohda, A. and Nishimune, Y. (2003) Proliferation and differentiation of spermatogonial stem cells in the w/wv mutant mouse testis. Biology of reproduction, 69, 1815-1821.
9. Yan, W. (2009) Male infertility caused by spermiogenic defects: lessons from gene knockouts. Molecular and cellular endocrinology, 306, 24-32.
10. Cooke, H.J. and Saunders, P.T. (2002) Mouse models of male infertility. Nature reviews. Genetics, 3, 790-801.
11. Zhu, Z., Li, C., Yang, S., Tian, R., Wang, J., Yuan, Q., Dong, H., He, Z., Wang, S. and Li, Z. (2016) Dynamics of the Transcriptome during Human Spermatogenesis: Predicting the Potential Key Genes Regulating Male Gametes Generation. Scientific reports, 6, 19069.
12. Miyamoto, T., Hasuike, S., Yogev, L., Maduro, M.R., Ishikawa, M., Westphal, H. and Lamb, D.J. (2003) Azoospermia in patients heterozygous for a mutation in SYCP3. Lancet, 362, 1714-1719.
13. Christensen, G.L., Ivanov, I.P., Atkins, J.F., Mielnik, A., Schlegel, P.N. and Carrell, D.T. (2005) Screening the SPO11 and EIF5A2 genes in a population of infertile men. Fertility and sterility, 84, 758-760.
14. Jamsai, D. and O'Bryan, M.K. (2011) Mouse models in male fertility research. Asian journal of andrology, 13, 139-151.
15. Zhang, Y., Zhong, L., Xu, B., Yang, Y., Ban, R., Zhu, J., Cooke, H.J., Hao, Q. and Shi, Q. (2013) SpermatogenesisOnline 1.0: a resource for spermatogenesis based on manual literature curation and genome-wide data mining. Nucleic acids research, 41, D1055-1062.